Synthesis and Structural Study of Titanium (IV) Complexes Derivative from 2, 6-; 3,5-and 2,4-dihydroxybenzoic Acid Molecular Modelling Approach
Abstract
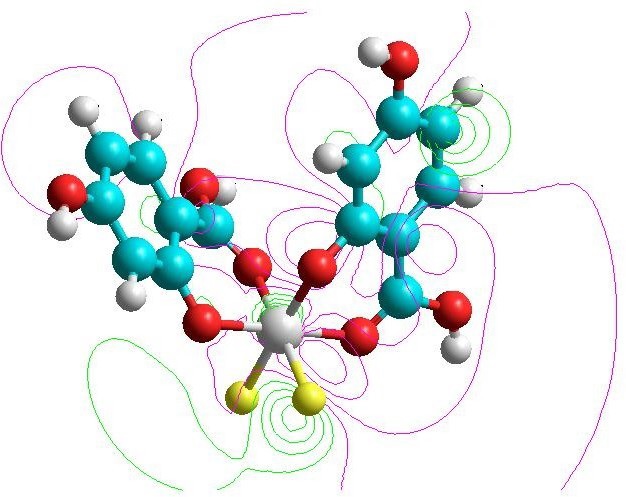
Complex fluorinated titanium (IV) derivative of 2, 6- dihydroxybenzoic acid (L1), 3,5-dihydroxybenzoïc acid (L2) and 2,4-dihydroxybenzoïc acid (L3) are known for their great interest in the dental oral area. They are widely used in the prevention of dental caries. These complexes by spectroscopic methods of analysis (UV-Vis., IR) are synthesized and then characterized. Since the reactivity of a complex depends strongly on its geometry’s stability, the most stable geometry of these complexes using molecular modelling is then determined. In order to calculate the steric energy, bond lengths, bond angles and torsion angles data molecular mechanics calculations (MM) using Hyperchem software is performed. For this purpose, the well-known Density Functional Theory calculations (DFT) to study the HOMO and LUMO properties of this complex is used. Both experimental and theoretical showed a distorted octahedral structure around the metal ion Ti (IV).
Full Text:
PDFReferences
M.J.M. Campbell, Coord. Chem. Rev., 1975, 16, 279.
W. Henderson, L J. McCaffreyu and B.K. Nicholson, J. Chem. Soc. Dalton Trans., 2000, 16, 2753.
A.E. Liberta and D.X. West, Biometals,1992, 5, 121.
E.J. Corey and J.C. Bailar, J. Am. Chem. Soc., 1959, 81, 2620.
Z. Zizi, A.Benghalem, L. Benmeni, S.M. Al-Asquar and M. M. Mostafa, Int. J. Chem., 2007, 17,57.
Z. Zizi, M. M. Mostafa and A. Benghalem, Mansoura J. Chem., 2005, 32(1), 167.
Z. ZIZI; A. Benghalem; A. M. Summam and M.M. MOSTAFA, Inter. J. Chem., 2004, Vol.14(4).
I. Aoues and Z. Zizi, Bull. Chem. Soc. Ethiop., 2018, 32(3), 571.
R.D. Hancock, Acc. Chem. Res., 1990, 23, 253.
R. Hoffman, J. Chem. Phys., 1963, 39,1397.
A.B. Anderson and R. Hoffman, J. Chem. Phys., 1974, 60, 4271.
G. Calzaferri, L. Fross and I. Kamber, J. Chem. Phys., 1989, 93, 5366.
E. Clementi and C. Roetti, Roothan- Hrtree-fock Atomic Wavefunction, Atomic Data and Nuclear Data Tables, 1974,14, 177.
M. H. Prosnec, C. Janiak and H.H. Brintzinger, Organometallics, 1992, 11, 4036.
A. F. Jalbout, B. Trzaskowski, A. J. Hameed, J. Organomet. Chem., 691 (2006) 4589.
A.F. Jalbout, A. J. Hameed, B. Trzaskowski, J. Organomet. Chem., 2007,692, 1039.
Y. Qian, J. Huang, M.D. Bala, B. Lian, H. Zhang and H. Zhang, Chem. Rev., 2003, 103, 2633.
R. Beckhause and C. Santamaria, J. Organomet. Chem., 2001, 617, 81.
E. Manek, D. Hinz and G. Meyer, Coord. Chem. Rev., 1997, 164, 5.
J.C. Vites and M.M. Lynam, Coord. Chem. Rev., 1995, 138, 71.
R.O. Duthaler and A. Hafner, Chem. Rev., 1992, 92, 807.
B.K. Keppler, C. Friese, H.G. Moritz, H. Vongerichten and E. Vogel, Struct. Bond., 1991, 78, 98.
M.J. Go, J.M. Lee, K.M. Lee, C.H. Oh, K.H. Park, S.H. Kim, M. Kim, H.R. Park, M.H. Park, Y. Kim and J. Lee, Polyhedron., 2014, 67, 286.
C. Finidori, World Intellectual Property Organization,WO/01/005797, 2001.
. A. Rajini, M. Nookaraju, I.A.K. Reddy, N. Venkatathri,Journal of Saudi Chemical Society., 2017, 21, S77–S85.
. P.P. Cobri, A.C. Massabni, A.G. Moreira, F.J. Medrano, M.G. Jasiulionisand C.M. Costa-Neto, Can. J. of Chem.,2005, 83(2), 104.
I. Brudzinska, Y. Mikata, M. Obata and C.S. Ohtsuki, Bioorg. Med. (Print),2004, 14(10), 2533.
C. Lee, W. Yang, R. G. Parr, Phys. Rev. B 37 (1988) 785; A.D. Becke, J. Chem. Phys. 98 (1993) 5648; T.A. Keith, R.F.W. Bader, Chem. Phys. Lett. 194 (1992) 1.
W. J. Geyary., Coord.Chem.Rev., 7(1971), 1-81.
D. X. West et L.D.Borowy, Trans.Met.Chem.,16(1991),5.
S. Geoge, Infrared and Raman Characteristic Group Frenquencies (Tables and Charts), 3ème edition, (2001), p305-311.
J. Giraudet, thèse de doctorat, Université Blaise Pascal, (2002) 22.
M.J. Go, J.M. Lee, K.M. Lee, C.H. Oh, K.H. Park, S.H. Kim, M. Kim, H.R. Park, M.H. Park, Y. Kim, J. Lee, Polyhedron, 67 (2014) 286.
L. Brohan et J.M. Nunzi, Action Concertée Energie (PE1),(2004).
J.L. Lamboy, A. Pasquale, A.L .Rheingold et E. Meléndez, Inorg. Chim.Acta., 360(6) (2007) 2115-2120.
Q. Wang, X. Xu et X. Wang, Acta Cryst., C49(1993),464-467.
I. Fleming, Frontier Orbitals and Organic Chemical Reactions, John Wiley & Sons, New York, (1976).
N. C. Handy, P. E. Maslen, R. D. Amos, J. S. Andrews, C.W. Murry, G. J. Laming, Chem. Phys. Lett.,197(1992),506-515.
Copyright (c) 2021 Turkish Journal of Materials

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Indexing:









